When Does 4cs in Norwood
Introduction
Hypoplastic left heart syndrome (HLHS) is a congenital heart disease that, while affecting but 1 out of every 4,344 births, accounts for xl% of all neonatal cardiac deaths (Mai et al., 2010). HLHS is characterized by left ventricular inflow and aortic outflow tract hypoplasia that limits cardiac output and is apace fatal if untreated. Therefore, HLHS patients must undergo a series of 3 surgical procedures to palliate these complex anatomical defects into a single ventricle system. Stage I palliation, or the Norwood procedure, is usually performed within the first week of life. The Norwood procedure consists of reconstructing the aortic arch and connecting it to the correct ventricle, then placing a systemic-to-pulmonary artery (PA) shunt to enable blood menses to the PAs. Options for the shunt include a modified Blalock-Thomas-Taussig shunt (mBTTs) from the innominate artery to the PAs (Figure 1A), a central shunt from the ascending aorta to the PAs, or a Sano shunt from the correct ventricle to the PAs. However, in this report it was decided to focus on the mBTTs arroyo as it is a common approach utilized for HLHS patients (Sano et al., 2004). Following stage I palliation, there are numerous reported complications, including reduced cardiac output, insufficient growth of the PAs, and stenosis at the shunt suture sites, all of which can impact mortality and hinder the success of the subsequent surgical procedures (Tweddell et al., 2002; Wells et al., 2005; Kawada, 2008). Despite surgical refinements of the Norwood process, the complications following phase I palliation lead to a mortality rate of 15–25% (Hornik et al., 2012). Stage I palliation is followed past two additional procedures: phase Ii, which consists of a superior cavopulmonary anastomosis [bidirectional Glenn (Figure 1B) or hemi-Fontan process] at 4–6 months of historic period, and stage Three, the total cavopulmonary anastomosis (Fontan procedure) at eighteen–48 months of age). At phase 2 palliation, the mBTTs is removed, and superior vena cava menses is directed to the PAs (Effigy 1B). Despite significant improvement over fourth dimension, the highest adventure for bloodshed is in the firsthand post-stage I and in the interstage period betwixt stage I and stage II (Mahle et al., 2000; Tweddell et al., 2002). While abundant hemodynamic data are obtained during pre-operative evaluation of pre-stage II (pre-S2) conditions, firsthand post-stage I (mail service-S1) data are rarely reported. Despite many efforts to closely monitor the stage I post-operative course for HLHS patients, in that location remains a paucity of critically of import hemodynamic data (specifically PA hemodynamic information). The issue of this limited understanding of PA physiology is farther magnified when considering PA stenosis occurs in nearly fifty% of HLHS patients following stage I palliation (Griselli et al., 2006; Aiyagari et al., 2014). In this work, nosotros seek to characterize the physiological mechanisms that tin potentially contribute to understanding complications following phase I palliation. By combining clinical information and computational fluid dynamics (CFD) tools, we gain insight on hemodynamics immediately following stage I palliation and characterize the development of key hemodynamic indices between phase I and stage Ii.

Figure 1. Anatomical configuration later on the (A) stage I Norwood procedure and (B) stage Ii Bidirectional Glenn (Migliavacca et al., 2002).
Despite previous utilise of CFD models to written report shunt placement, shunt designs, and various surgical configurations of HLHS patients, they oft contain numerous shortcomings. These models take assumed purely 0D (lumped parameter models) or 3D rigid wall approaches and are oft patient-specific models (therefore non reflecting population-based hemodynamic and anatomical characteristics) (Bove et al., 2003; Qian et al., 2010; Ceballos et al., 2012; Itatani et al., 2012; Moghadam et al., 2012; Baker et al., 2013; Arthurs et al., 2017; Fumero et al., 2017). Furthermore, these models are based solely on clinical data collected 4–five months subsequently initial palliation (pre-S2) leading to a limited understanding of the early on post-operative conditions HLHS patients experience. Upwardly to this point, at that place has not been a computational study that investigates the hemodynamics of HLHS patients immediately following phase one palliation. A computational fluid-structure interaction (FSI) model representing a broad patient population immediately post-S1 and through pre-S2, would lead to an enhanced agreement of the atmospheric condition and complications that HLHS patients experience. We seek to combine imaging and literature-based clinical data to develop computational models that are representative of HLHS patients for both post-S1 (within outset week after palliation) and pre-S2 (within days earlier stage II palliation) conditions. These computational tools may let us to gain detailed insights into the complex physiologic weather condition these patients feel between stage I and stage II palliation (Arthurs et al., 2017).
Materials and Methods
The development of both post-S1 and pre-S2 models required the careful compiling of clinical information and computational methodologies discussed herein. First, nosotros will describe the general modeling approach that was taken to construct both models. Then, we will talk over the clinical information that was measured and collected to influence both models. Finally, we will outline the steps required to construct, calibrate, and parameterize both post-S1 and pre-S2 models.
Overview
To construct geometries that stand for a broad population of HLHS patients who take undergone the Norwood procedure, we began by constructing a patient-specific model equally a representative baseline configuration (Figure 2A). Once this "template" model was constructed, morphological literature data was used to scale the geometry to reverberate HLHS patient anatomy immediately post-obit stage I palliation (mail-S1) and prior to stage II palliation (pre-S2) (Effigy 2B). Literature data reflecting post-S1 and pre-S2 hemodynamic conditions were applied to the corresponding models to simulate the complex hemodynamics these patients experience at both time-points. The outcome of this approach is a weighted-average (or "population-average") model that represents a typical HLHS patient at the two time-points of interest–mail-S1 and pre-S2. The written report was approved past the University of Michigan Board of Review.

Figure 2. Modeling approach used to reconstruct anatomy of representative HLHS patient geometry at 2 time-points–post-S1 and pre-S2. Anatomy was constructed from (A) patient-specific MRI data that was then (B) scaled to match literature-based morphological data for both time-points (Machii and Becker, 1997; Mahle et al., 1998; Malec et al., 2003; Fiore et al., 2011; Bellsham-Revell et al., 2013; Aiyagari et al., 2014). The slight degree of tapering at each anastomosis of the shunt tin can be observed.
Clinical Data
Patient-Specific Data
Clinical data of a 3-week old HLHS patient was acquired. At birth, this patient exhibited anatomical characteristics typical for HLHS patients (mitral and aortic atresia, non-restrictive atrial septal defect) and underwent the Norwood procedure at 4 days of age (Jonas et al., 1994). During stage I palliation, the surgeon found the patient's innominate artery suboptimal for shunt placement, and then the decision was fabricated to connect the systemic-to-pulmonary shunt from the ascending aorta to the master pulmonary artery (MPA) (see Figure 2A). 2 weeks after stage I palliation, magnetic resonance angiography (MRA) information were acquired to assess mail service-operative characteristics including PA stenosis, palliation of the aortic arch, and possible shunt occlusion. Furthermore, stage-contrast magnetic resonance imaging (PC-MRI) data were collected at the ascending aorta, transverse aortic arch, descending aorta, and bilateral PAs to appraise critical parameters such as cardiac output, pulmonary-to-systemic flow ratio (Qp:Qs), and valve regurgitation. Blood pressure measurements were nerveless using a brachial cuff concurrent with the MRA and PC-MRI examinations.
At the time of imaging information acquisition, the patient continued to exhibit anatomical characteristics of a typical HLHS patient with no boosted compromising anomalies (i.e., aortopulmonary collateralization, PA stenosis, shunt occlusion). Of note, the distal transverse aortic curvation of the patient was markedly dilated (Figure 2A). The proximal right PA was as well mildly angulated, a common finding in patients afterwards stage I palliation (Mahle et al., 1998).
Literature Population Information
In lodge to develop representative computational models of typical HLHS patients, the acquired patient-specific clinical data was carefully combined with literature information on anatomy (Figure 2) and hemodynamics (Tabular array 1) for the two time-points of interest: post-S1 and pre-S2 (Rychik et al., 2002; Maher et al., 2003; Mahle et al., 2003; Mair et al., 2003; Malec et al., 2003; Pizarro et al., 2003; Ballweg et al., 2005; Cua et al., 2006; Ghanayem et al., 2010; Fiore et al., 2011; Bellsham-Revell et al., 2013; Aiyagari et al., 2014). All literature values were based on patients born with HLHS who had undergone the Norwood procedure with a surgically placed mBTTs.
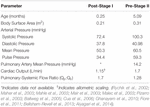
Table 1. Literature-based weighted means of hemodynamic parameters for post-S1 and pre-S2.
To obtain literature-based values, a detailed review of all available literature that included hemodynamic and morphological information of HLHS patients was conducted. Inclusion criteria included: (ane) patient populations presented with HLHS, (2) the use of the mBTTs during the Norwood procedure, (3) size of surgically placed shunt either 3.v mm or 4.0 mm, and (iv) reporting morphological and/or hemodynamic data within the first week post-S1 and/or at pre-S2 follow-upward. Exclusion criteria included: (i) report population overlapped with another study and (two) patient population presented with coexisting cardiac anomalies. The search strategy yielded 15 included studies (Tables ii, 3).

Table ii. Hemodynamic and morphological literature data for healthy patients (left) and HLHS postal service-S1 patients.

Table iii. Hemodynamic and morphological literature data for patient pre-S2.
Post-obit the Norwood procedure, mail service-S1 data was nerveless during the post-operative hospitalization (mean historic period of 7 days) (Table 2). Hemodynamic information were obtained from brachial cuff pressure, cardiac catheterization, Doppler echocardiography, and oxygen saturation of blood samples (Rychik et al., 2002; Mahle et al., 2003; Mair et al., 2003; Malec et al., 2003; Pizarro and Norwood, 2003; Cua et al., 2006; Ghanayem et al., 2010). Reported hemodynamic data included systemic arterial pressure and Q p :Q s (Table two). Morphological data was obtained using echocardiography (Machii and Becker, 1997; Mahle et al., 1998; Pettersen et al., 2008). Regions of native tissue (supra-aortic arteries, descending aorta, LPA, and RPA) were assumed to exhibit normal morphological characteristics of children less than ane-calendar month of age (Machii and Becker, 1997; Pettersen et al., 2008). This assumption cannot be applied to the reconstructed region of the aorta. Measurements of the reconstructed arch in HLHS patients during the mail service-operative menstruation following the Norwood procedure were applied from the ascending aorta to the transverse arch (Mahle et al., 1998).
Pre-S2 data was collected during pre-operative follow-upwards for stage Ii palliation (mean age of 5.1 months). Hemodynamic information was obtained from cardiac catheterization and PC-MRI (Maher et al., 2003; Mahle et al., 2003; Mair et al., 2003; Malec et al., 2003; Ballweg et al., 2005; Fiore et al., 2011; Bellsham-Revell et al., 2013; Aiyagari et al., 2014). Reported hemodynamic data included systemic arterial pressure level, PA mean pressure, cardiac output, and Qp:Qs (Table 3). Morphological information was obtained using MRA and echocardiography (Machii and Becker, 1997; Ballweg et al., 2005; Fiore et al., 2011; Bellsham-Revell et al., 2013; Aiyagari et al., 2014). The descending aorta and supra-aortic arteries were causeless to showroom normal morphological characteristics of children from 1-calendar month to less than 1 year in age (Machii and Becker, 1997). Post-obit the Norwood procedure, the PAs are known to feel unfavorable hemodynamic conditions leading to contradistinct growth, and so pre-S2 measurements of the LPA and RPA were applied (Ballweg et al., 2005; Bellsham-Revell et al., 2013; Aiyagari et al., 2014). Measurements of the reconstructed aortic arch in HLHS patients during pre-S2 assessment were applied from the ascending aorta to the transverse curvation (Bellsham-Revell et al., 2013).
Statistical Analysis
Weighted mean values of collected literature-based hemodynamic and morphological parameters were calculated using the fixed effect model (Tabular array 1; Borenstein et al., 2010). By considering sample size and standard deviations of each study, the fixed effect model applies more weight to studies that report more than precise data, therefore providing information more than representatives of typical HLHS patients for the two time-points studied. Some studies within the literature review did not report the standard deviation of measured parameters. In this instance, a method commonly practical to approximate standard divergence (or variance) from reported median, range, and sample size was used (Hozo et al., 2005). All literature-based weighted means of hemodynamics for both post-S1 and pre-S2 can be found in Table one.
CFD Simulations
Computational modeling tools were applied to calculate the hemodynamic conditions of HLHS patients following the Norwood procedure. CFD is a well-established methodology that enables the calculation of the velocity and pressure fields of an incompressible fluid by solving the Navier–Stokes equations. In this written report, to perform FSI simulations nosotros must define: (i) geometric representation of the regions (claret vessels) of interest, (2) inflow and outflow boundary conditions representing pressure and velocity of typical HLHS patients, and (3) cloth properties for all vessel walls.
Geometric Modeling and Mesh Generation
Representative FSI models of post-S1 and pre-S2 HLHS patients were constructed using the cardiovascular modeling and blood menstruation simulation software bundle CRIMSON1 (Arthurs et al., 2020). A patient-specific model was first reconstructed from the MRA data (Figure 2A). This model included the ascending and descending aorta, left and correct PA, left and right common carotid arteries, left and right subclavian arteries, and the surgically placed systemic-to-PA shunt. After reconstructing the patient-specific anatomy, the geometry was scaled to reverberate literature-based morphological data measured mail-S1 and during the standard clinical follow-up pre-S2 (Figure 2B; Machii and Becker, 1997; Mahle et al., 1998; Malec et al., 2003; Fiore et al., 2011; Bellsham-Revell et al., 2013; Aiyagari et al., 2014). Furthermore, the key shunt of the patient-specific geometry was replaced with a mBTTs shunt as this configuration is utilized more frequently in current practice. When making a significant alter to the geometry, such as this, it is key to remain consistent with all remaining steps of the modeling process to avert whatever misleading simulation results. Therefore, all data used during scaling and calibration were nerveless from patients with the specific mBTTs surgical configuration. Of note, the PA and descending aorta of the post-S1 model reflects diameters of healthy subjects equally these regions of tissue are not reconstructed during the Norwood procedure and accept not undergone significant remodeling as a event of palliation. The mBTTs was modeled with a diameter of 3.5 mm as this was the well-nigh common shunt size reported in the literature data. Furthermore, a small degree of tapering was modeled at each anastomosis of the shunt. "Pinching" at the shunt suture line has been reported to immediately impose a x% diameter reduction at each anastomosis (Dobrin et al., 1998; Figure ii). This diameter reduction was applied to the post-S1 model. During calibration of the pre-S2 model, it was found that a 17% diameter reduction (bringing the shunt diameter to 2.9 mm) was required to reproduce PA hemodynamics found in the literature. This additional degree of pinching in the pre-S2 model could be attributed to intimal hyperplasia commonly occurring at suture sites in mBTTs patients, and is in line with previously reported values (Gladman et al., 1997; Wells et al., 2005).
Isotropic mesh generation using linear tetrahedral elements resulted in 505,964 elements for the post-S1 model and 743,456 elements for the pre-S2 model. Preliminary simulations were performed with these meshes and gradient-based mesh adaptive techniques were utilized to refine the finite chemical element mesh in regions of high velocity gradients (Sahni et al., 2006). This produced adapted meshes consisting of i,167,047 and 1,623,130 elements for the mail-S1 and pre-S2 models, respectively. The results reported in this study used these finer meshes.
Boundary Condition Specification
Each model consisted of one inlet (ascending aorta) and seven outlets (all other modeled vessels). A multi-domain modeling approach was utilized to characterize blood menses. This method couples the non-linear incompressible three-dimensional Navier–Stokes equations describing velocities and pressures in 3D reconstructed vessels with a series of 0D lumped parameter networks (LPN) that capture distal hemodynamic behavior (resistance and compliance) besides equally ventricular part (Figure 3). This approach has been successfully implemented to report single ventricle physiology (Moghadam et al., 2012; Arthurs et al., 2017). The Cherry Netlist Editor Boundary Condition Toolbox enables the introduction of dynamic, customizable three-element Windkessel models at the outlets and a heart model at the inlet (Figure 3A), and even coupling disconnected 3D domains (due east.g., separate systemic and pulmonary geometries) in a closed-loop manner (Silva Vieira et al., 2018). Three-element Windkessel models were practical to each outlet to represent the resistance and compliance of the distal vasculature (Figure 3C; Vignon-Clementel et al., 2010). A custom 0D lumped parameter heart model was applied at the inflow of the neo-aorta (Figure 3B; Lau and Figueroa, 2015). The heart model included a series of 0D components that represented the right atrial and neo-aortic valves and the contraction of the correct ventricle (Effigy 3B). The contraction and relaxation of the right ventricle was simulated using time-varying elastance function (Figure 3B). The valves were modeled as diodes and only permitted forward flow.
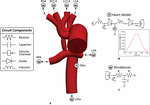
Effigy 3. (A) The 3D-0D multi-domain framework utilized to simulate hemodynamics in both post-S1 and pre-S2 models. The geometry corresponding to the pre-S2 model is shown. Outlets with "West" represent to a Windkessel purlieus condition (B). Outlet with "H" corresponds to the heart model boundary status. (B) 0D eye model coupled to the ascending aorta inlet face. A plot of fourth dimension-varying elastance to model the contraction and relaxation of the right ventricle (RV) is included. (C) Iii-chemical element Windkessel model representing the distal vasculature applied to all outlets. Abbreviations: Fifty, inductor; D, diode; RPA, right pulmonary avenue; LPA, left pulmonary artery; P, pressure; Q, period; RV, right ventricle; RA, right atrium; R p , proximal resistance; R d , distal resistance; C, compliance; AVV, atrioventricular valve; NAoV, neo-aortic valve. Calibrated LPN parameter values are listed in Table 4.
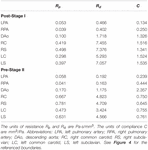
Table iv. Tuned component parameters for the Windkessel models at the outlets of both the post-S1 and pre-S2 models.
Fluid-Structure Interaction
Virtually CFD studies of HLHS patient hemodynamics accept assumed rigid walls for all vessels. While this assumption can produce a good estimate of the velocity field, it has a significant bear on on the pressure field and estimates of the LPN parameter values that could atomic number 82 to misleading results. In this work, the "Coupled Momentum" FSI method was used (Figueroa et al., 2006). This required fabric properties, specifically linearized stiffness and thickness to be specified for each vessel. A uniform vessel thickness of ane.5 mm was practical to the aorta and PAs for both models, and a spatially varying thickness of 15% of the local vessel radius was specified for the aortic upper branches (van Meurs-van Woezik et al., 1983; Roccabianca et al., 2014). Linearized stiffness was determined using the patient-specific MRA information on dynamic luminal surface area (systolic to diastolic range), and pressure level cuff data (section "Patient-Specific Data"), meet Effigy 4B (Hirai et al., 1989). Stiffness values were kept constant for both models, given that vessel wall properties do not significantly change inside the first year of life (Figure 4C; Senzaki et al., 2002). The material properties determined are in line with reported values for HLHS patients post-obit the Norwood process (Cardis et al., 2006). The reconstructed region of the aorta (transverse arch) exhibits the highest values of stiffness as information technology primarily consists of constructed graft material. The ascending and descending aorta and PAs accept lower stiffness since they consist of native tissue, and, every bit a result, are more distensible (Effigy 4C). Finally, the shunt was assumed to exist rigid.
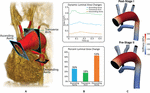
Figure iv. (A) Reconstructed geometry with volume-rendering of MRA information and three different PC-MRI planes. (B) Plot of dynamic luminal expanse changes and percentage luminal area changes at the ascending aorta, transverse arch and descending aorta PC-MRI planes. (C) Textile properties applied to both the post-S1 and pre-S2 models. Stiffness values were calculated based on patient-specific pressure measurements and dynamic luminal area changes measured from MRI data (Hirai et al., 1989).
Boundary Condition Parameterization
The parameterization of the multi-domain model seen in Figure 4 consisted of two main steps. In Stride 1, a flow waveform was prescribed at the aortic inflow, and a calibration of the outflow co-operative's LPN parameters was performed. In Step ii, upon calibration of outflow LPN parameters, a custom heart model was prescribed at the aortic inflow and calibrated (Xiao et al., 2013).
Step 1
The measured PC-MRI aortic inflow waveform was scaled to lucifer the cardiac output institute in literature for both the postal service-S1 and pre-S2 models. Calibration of LPN parameters was iteratively performed by running simulations on sixty cores in the Academy of Michigan high-performance calculating cluster ConFlux. Simulations were run until cycle-to-cycle periodicity was reached in the pressure field, which typically took five cardiac cycles. Each simulation took approximately 48 h to complete depending on mesh size. Initial parameters of Windkessel elements (resistance and compliance) of all outlets were calculated using the iterative method described by Xiao et al. (2013). Then, total resistances of the Windkessel elements for both models were calibrated until the mean pressures and hateful flow at each outlet matched within x% of the data constitute in literature. Then, the Windkessel compliances were calibrated until the desired pulse force per unit area was achieved within ten%.
Stride 2
The prescribed inflow was replaced by a custom 0D lumped parameter heart model at the inflow of the neo-aorta. The correct atrial pressure level (P RA ) and parameters of the elastance function were adjusted until cardiac output was matched within 5% of values plant in literature (Arthurs et al., 2017). Once the cardiac output was matched, small-scale adjustments were made to the outflow boundary weather until all hemodynamic parameters were matched inside 10% of the data plant in literature. The final numerical values for the components of the LPN outflow Windkessel and the heart model are presented in Tables 4, 5, respectively. The resultant Windeasel parameters for the pre-S2 case compare well to previously published values (Arthurs et al., 2017). Unfortunately, there are no previously published values for the post-S1 case to compare resultant Windkessel parameters.
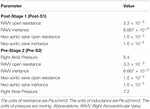
Table 5. Tuned component parameters for the heart models at the inlets of both the post-S1 and pre-S2 models.
Results
Calibration Confronting Hemodynamic Data
Model calibration was initially attempted assuming rigid walls; however, we were uncapable unable of matching hateful pressure and flow data while simultaneously matching pulse pressure. In one case utilizing the more physiologically relevant approach of deformable walls, nosotros were able to match all available population-based hemodynamic literature information. Both post-S1 and pre-S2 models were successfully calibrated to closely lucifer literature-based hemodynamics on systemic pressure, cardiac outflow, Qp:Qs and, for the pre-S2 model, PA mean pressure. Simulated hemodynamic indices were within 8% of the literature-based information (Table ane) for both models (Figure five).
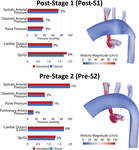
Figure 5. Left: Comparison of fake results (ruddy) and literature-based clinical data (bluish) of hemodynamic indices for mail service-S1 and pre-S2 models. All indices were matched inside 10% of literature values. Correct: Book renders of FSI simulations velocity maps.
Simulated Menses and Pressure level Waveforms
Effigy half-dozen shows a comparison of the simulated pulsatile flow waveforms at all outlets for both the mail-S1 and pre-S2 models. The hateful menstruation through all outlets increased from mail-S1 to pre-S2. This is to exist expected as, in agreement with literature data, at that place was a 38% increase in cardiac output between stage I and phase Ii (Table ane). Despite Qp:Qs decreasing from post-S1 (1.8) to pre-S2 (1.3), the mean PA flow increased from mail service-S1 (0.75 L/min) to pre-S2 (0.97 Fifty/min). The hateful flow observed at the correct common carotid and subclavian arteries is lower compared to that observed at the left common carotid and subclavian arteries. The waveforms within the right common carotid and correct subclavian arteries nowadays high-frequency disturbances in top systole, which are absent on their left side counterparts. This tin can be attributed to the "run-off" of period through the mBTTs to the pulmonary circulation that is commonly seen within HLHS patients (Kawada, 2008). Diastolic backflow is observed in the descending aorta and the supra-aortic vessels for both models. The overall alter in the shape of the menstruation waveforms from post-S1 to pre-S2 is subtle.
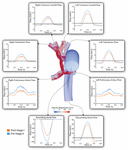
Figure 6. Faux pulsatile flow waveforms at all boundaries of the 3D domain for both post-S1 (orange) and pre-S2 (blue) models. All outflows are plotted as positive and all inflows are plotted equally negative. Note that three different y-centrality scales have been used: one for the descending aorta, another for the ascending aorta and a 3rd for the balance of the outlets. A volume rendering of velocity magnitude for the pre-S2 model is shown during peak-systole (t = 0.21 due south).
Figure 7 shows a comparison of the simulated pulsatile pressure waveforms at all outlets for both the mail service-S1 and pre-S2 models. While the simulated hateful systemic arterial pressure increased from post-S1 (47.five mmHg) to pre-S2 (60 mmHg), the PA mean force per unit area decreased substantially from approximately 22 to 14 mmHg. Lower mean pressures and subtle high-frequency oscillations in peak systole were observed at the right mutual carotid and subclavian arteries compared to the left common carotid and subclavian arteries. This is similar to the "run-off" phenomenon observed in mean menstruum received by the correct common carotid and subclavian arteries (Kelleher et al., 2006).
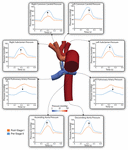
Effigy vii. False pulsatile pressures at all boundaries of the 3D domain for both mail service-S1 (orange) and pre-S2 (blue) models. Arrows indicate whether force per unit area increased or decreased from post-S1 to pre-S2. Note that two different y-centrality scales have been used: one for the LPA and RPA, and a second for the rest of the outlets. A pressure coloring on the surface of the pre-S2 model is shown during superlative-systole (t = 0.21 s).
Disturbed Pulmonary Avenue Hemodynamics
High-frequency oscillations in pulmonary menstruum, indicative of hemodynamic disturbances (Tossas-Betancourt et al., 2020), were observed in both postal service-S1 and pre-S2 models. Disturbed PA flow is farther illustrated in Figure 8. As seen in the velocity streamlines, the parallel velocity streamlines within the shunt become highly disturbed in the region where the shunt meets the main PA, propagating these disturbances throughout the PAs.
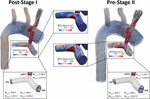
Figure 8. Velocity streamlines for both postal service-S1 and pre-S2 models during acme systole (t = 0.21 s). Disturbed menses patterns are prominent at the mBTTs-PA anastomosis and propagate throughout the PAs. Surface plots of WSS (center) in the PAs are presented for both models. There are concentrations of high WSS where the mBTTs flow impacts the wall of the PA. The WSS experienced in the PAs slightly increases during the interim period between stage I and II. Finally, velocity profiles at the mBTTs, LPA, and RPA (bottom) and their corresponding fourth dimension boilerplate and acme systolic Reynolds numbers. Loftier Reynolds numbers (>ii,000) are indicative of turbulence and can exist seen inside the mBTTs and PAs. These Reynolds numbers increase from post-S1 to pre-S2.
Wall shear stress (WSS) is some other key regulator of vascular biology. Information technology is known that spatially disturbed WSS may lead to pathological remodeling (Dolan et al., 2013). At post-S1 the PAs experience high values of WSS and continue to increase throughout the pre-S2 period.
Disturbed PA hemodynamics are further supported by large Reynolds numbers within the shunt and left and right PAs (Figure 8), indicating transitional/turbulent menstruum regimes. We calculated fourth dimension average (Re avg ) and peak systolic (Re max ) Reynolds numbers at the shunt and each of the PA outlets. Peak systolic Reynolds numbers within the mBTTs (3,314.9 and iv,305.six for post-S1 and pre-S2, respectively) were indicative of turbulence. It is too noteworthy that all calculated Reynolds numbers increase from postal service-S1 to pre-S2 indicating the hemodynamics become more than disturbed throughout the pre-S2 menses. In this newspaper, we adopted a "Direct Numerical Simulation" arroyo, and avoided the use of turbulence models. Convergence of the reported Reynolds number estimates would crave much more than refined finite element meshes yet and is therefore exterior the scope of this piece of work.
Mesh Independence and Numerical Accurateness
To ensure that the disturbed hemodynamics observed in the PAs were not a result of numerical fault, we performed a mesh independence analysis. Three increasingly fine isotropic linear tetrahedral element meshes were generated for both Post-S1 and Pre-S2 models. Mesh resolution was increased past incrementally decreasing the global element size (L) for each mesh generated–coarse (50 = 0.v mm), fine (Fifty = 0.3 mm), and very fine (50 = 0.2 mm). The fibroid, fine, and very fine meshes of the Postal service-S1 model consisted of 360,027 elements, ane,501,846 elements, and four,793,832 elements, respectively (Effigy 9). The fibroid, fine, and very fine meshes of the Pre-S2 model consisted of 544,754 elements, ii,244,714 elements, and 7,221,928 elements, respectively (Figure 9). Numerical results were deemed mesh-independent when difference in regional pressure was <ane% between two successive meshes.
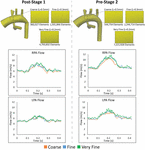
Figure 9. Top: The diverse isotropic linear finite chemical element meshes generated for the mesh-independence analysis for both Postal service-S1 and Pre-S2 models. Finer meshes are generated past incrementally decreasing the global element size (L). Bottom: Results from the mesh independence analysis. Flow at the LPA and RPA are plotted for both Mail-S1 and Pre-S2 at each mesh size–fibroid (orange), fine (blue), and very fine (green). Equally seen in each plot, at that place is piffling alteration in mean catamenia values in the PAs and the loftier-frequency oscillation is present in all cases.
Regional pressure level differences were <1% between the fine and very fine meshes for both Post-S1 and Pre-S2 models. This level of convergence inside the pressure level field ensures that these models are mesh independent. Furthermore, the high-frequency oscillations within the PA flow field remain nowadays for all levels of refinement for both Post-S1 and Pre-S2 models (Figure 9). This indicates that the high-frequency components are not a result of numerical error but represent a true concrete phenomenon. Information technology is worth noting that as the mesh becomes more refined, the high-frequency components become more pronounced in both models.
Discussion
Summary
Fluid-structure interaction models of post-S1 and pre-S2 HLHS patients were constructed and calibrated to match in vivo hemodynamic and morphological data found in literature. Ultimately, we sought to leverage imaging data, literature data, and computational modeling to enhance our express understanding of HLHS hemodynamics immediately after stage I palliation. Furthermore, by developing a calibrated pre-S2 model, nosotros were able to describe central hemodynamic changes between the ii surgical stages. The development of key hemodynamic indices (pressure, flow, resistance, cardiac output, and Qp:Qs ratio) is given in Effigy 10.

Figure 10. The evolution of hemodynamic indices from ∼7 days of age at mail-S1 to ∼v months of age at pre-S2.
Evolution of Hemodynamic Indices From Stage I to Stage II
Pulmonary avenue pressure level is a key unknown hemodynamic parameter at post-S1 mainly due to the need for invasive cardiac catheterization and the risks involved with introducing a catheter through the systemic-to-PA shunt. This work has given usa insight into mail-S1 force per unit area. By matching all available hemodynamic and anatomical data within 10%, and using literature data for typical post-S1 shunt "pinching," the mail service-S1 model revealed a PA mean force per unit area of 22 mmHg (Figure viii). This elevated PA mean force per unit area is a key finding of this paper and is greater than the pre-S2 mean pressure level (14 mmHg). Ideally, we could corroborate this finding with previously published values of post-S1 PA pressure and hemodynamic; all the same, this data has not been published on up to this point.
The hateful systemic and pulmonary resistances decreased from post-S1 to pre-S2: from 37.1 to 31.2 Wood units in systemic resistance, and from 14.8 to 3.8 Forest units in pulmonary resistance. This is in line with what is seen in growing neonates, since distal vascular resistance decreases as vessel and lung maturation ensues (Lucas et al., 1961). This decrease in pulmonary resistance probable drives the increase in mean PA menstruum at pre-S2 (0.97 Fifty/min, 25% increase from 0.75 L/min at mail service-S1), despite the larger resistance due to the smaller anastomosis bore in pre-S2 (2.nine mm) relative to mail service-S1 (three.15 mm) (run across section "Geometric Modeling and Mesh Generation"), which would tend to limit pulmonary flow. The decrease in systemic resistance resulted in a proportionately larger increment in systemic flow and therefore a more counterbalanced Qp:Qs ratio (from 1.8 to i.three), which nonetheless remained at a sustainable value for lung perfusion at pre-S2.
The compliance of the head and neck vessels (RC, RS, LC, and LS) decreases over time while the compliance of the DAo, LPA, and RPA increases. Broadly, compliance is the alter in volume divided past the change in pressure. ΔV is the integral of the flow, which is given by changes in the Qp:Qs ratio. As the body grows, a greater percentage of the cardiac output is directed to the lower body (DAo). Therefore, the ΔV increases, and so does the compliance. Every bit for the LPA and RPA, the ΔP goes downwards and this also explains why the compliance increases over time. Furthermore, the DAo has more compliance than the LPA and RPA. This can as well be understood through the relationship between compliance, changes in volume, and changes in force per unit area. For the pre-S2 example, in item, where data is bachelor for ΔP and for the volumes going to DAo and each of the PAs, the calibrated values of distal compliances for the different branches of the model (which let to match the bachelor population data) practice suggest that the total compliance distal to the aorta in the systemic circulation is larger than each of the distal compliances of the PAs. This trend has also been observed in previous computational analyses of HLHS patients (Arthurs et al., 2017).
Finally, despite HLHS patients experiencing volatility within the hours post-obit phase ane palliation, changes of macroscopic hemodynamic information used to calibrate the simulated models (aortic claret force per unit area, pulmonary-to-systemic flow ratio, etc.) are often either non statistically significant (Rychik et al., 2000; Pizarro and Norwood, 2003; Cua et al., 2006) or stabilize after day two (Mair et al., 2003). When available, data was collected at 48 h post-procedure.
Disturbed Pulmonary Artery Hemodynamics
The observed elevated PA hateful pressure, disturbed menses, and WSS maps suggest that HLHS patients are exposed to suboptimal conditions immediately after surgical reconstruction. The oscillatory hemodynamics are also present at the fourth dimension of pre-S2 assessment, indicating that these conditions are experienced throughout the interim menses betwixt phase I and Ii (Figure half dozen). The elevated values of WSS in the PAs are the result of the high flows through the shunt necessary to ensure adequate PA menstruation throughout the pre-S2 period, every bit the shunt becomes relatively smaller compared to the somatic growth of the patient; this could exist a key contributor to complications following stage I palliation.
Dubiety of Anastomosis Geometric Definition
Calibrated analyses (i.due east., reproducing all available flow data) performed in post-S1 and pre-S2 geometries with perfectly unrestricted shunts (uniform iii.five mm diameter without "pinching" at the anastomoses), rendered PA mean pressures of 40 and 24 mmHg, respectively. With the calibrated models producing such high pressures, it was adamant that nosotros were not capturing some aspect of shunt geometry that resulted in an additional pressure decrease from the systemic to the pulmonary circulation. Thus, it was decided that an alteration in shunt geometry was required to produce such additional pressure drop. Literature studies revealed that immediately following suturing of a PTFE graft to a native vessel, at that place is frequently a small caste of pinching at each anastomosis (Dobrin et al., 1998). This pinching becomes even more severe during the interim period between stages I and 2 as a shunt stenosis begins to develop at each anastomosis (Gladman et al., 1997; Wells et al., 2005).
With PA hemodynamic data available for pre-S2, determining the degree of pinching at the anastomosis constituted an changed trouble. From population-based data, the required pressure decrease across the shunt (60.v–14.3 = 46.iii mmHg, see Tabular array one) was known, so the degree of pinching was parametrically altered until the desired pressure drop was attained. It was found that a 17% subtract in bore at each anastomosis was required to reproduce population-based PA hemodynamics. This caste of diameter reduction is in line with what is commonly seen in patients who experience shunt stenosis (Gladman et al., 1997; Wells et al., 2005). Since there is no population-based PA hemodynamic data bachelor for postal service-S1, the caste of shunt pinching was modeled purely based on the available data on avenue-to-PTFE graft anastomoses institute in literature (Dobrin et al., 1998). Information technology was institute that when suturing a PTFE graft to a native vessel in that location is a ∼10% decrease in shunt diameter. This diameter reduction was applied to the post-S1 model.
Limitations
Our study has a few of limitations. First, the starting point of the study was paradigm data corresponding to a cardinal shunt patient, instead of a mBTT patient. Given that the purpose of the image data was to build a model in 3D space that would be afterwards heavily modified to reflect population average dimensions, the quality of the image information (and not the type of shunt) was the most important cistron considered when choosing the MRI information. 2nd, this written report simply investigated the Norwood procedure with a three.5 mm mBTTs. Although this surgical configuration and shunt size is widely utilized across many clinical centers; in reality, there are numerous different shunt sizes and surgical configurations (i.e., Sano shunt, key shunt) that could impact the outcome of the data presented. It is our hope that the presented simulations requite insight into this specific patient "class," but also a deeper understanding of what post-operative conditions could look like for HLHS patients undergoing any blazon of surgical approach. This warrants further investigation and the development of representative models of HLHS patients with other surgical configurations to gain a comprehensive agreement of weather condition following the Norwood process. Third, average material properties (i.e., vessel stiffness, wall thickness) were applied circumferentially, and therefore did not capture the spatial variation in stiffness in the reconstructed region of the aorta. Ideally, the much stiffer graft material backdrop would just be applied on the respective location of the reconstructed aorta and the balance would exist considered native tissue. Quaternary, we've shown mesh-independence in both the menstruum and pressure fields of our models; however, we have not shown mesh-independence for other hemodynamic quantities (i.e., WSS). Since the hemodynamics (and thus the WSS) are essentially disturbed for both post-S1 and pre-S2 cases, the models are likely to be subject to considerably deadening convergence rates in the WSS field (Les et al., 2010). In this study, our quantities of interest are flow and pressure, and for those nosotros have demonstrated adequate mesh independence in our results. Finally, the fixed effect model requires a standard deviation for computing weighted averages. Withal, some studies inside the literature review did not report the standard divergence of measured parameters. In this instance, a method commonly applied to guess standard deviation (or variance) from reported median, range, and sample size was used (Hozo et al., 2005).
Determination
In this study, we combined imaging data, population data, and computational modeling to enhance the current understanding of hemodynamics following the Norwood procedure. Following reconstruction, patients are immediately exposed to suboptimal hemodynamic conditions–elevated PA pressure level, oscillatory hemodynamics, and high WSS. Many of these conditions are however present at the time of stage 2 palliation. In the future, we seek to alter shunt design and configuration to minimize the degree of menstruum disturbances. We hypothesize that minimization of these flow disturbances would lead to more than favorable hemodynamics in the interim menses between surgical stages and therefore improve the outcomes of HLHS patients.
Information Availability Statement
The raw data supporting the conclusions of this article volition exist made available by the authors, without undue reservation.
Ethics Argument
The studies involving human participants were reviewed and approved by Academy of Michigan Medical Schoolhouse Institutional Review Board (IRBMED). Written informed consent from the participants' legal guardian/next of kin was non required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
JP wrote the manuscript, created the figures, performed and analyzed the literature review, built the geometric models, ran the simulations, processed data, and analyzed simulation results. JL acquired clinical information and assisted in manuscript preparation. AS and CF provided analysis of literature review and assisted in manuscript training. RG, CF, and AS developed concepts and assisted in manuscript preparation. CF provided analysis of simulation results. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Woodson Acceleration Grant and the Helen L. Kay Charitable Trust Grant. Computing resources were provided by the National Scientific discipline Foundation [grant 1531752] Conquering of Conflux.
Conflict of Interest
The authors declare that the enquiry was conducted in the absence of any commercial or fiscal relationships that could be construed as a potential conflict of interest.
Footnotes
- ^ www.cherry.software
References
Aiyagari, R., Rhodes, J. F., Shrader, P., Radtke, Due west. A., Bandisode, V. M., Bergersen, L., et al. (2014). Touch of pre-stage II hemodynamics and pulmonary artery anatomy on 12-month outcomes in the pediatric center network unmarried ventricle reconstruction trial. J. Thorac. Cardiovasc. Surg. 148, 1467–1474. doi: 10.1016/j.jtcvs.2013.10.057
PubMed Abstract | CrossRef Full Text | Google Scholar
Arthurs, C. J., Agarwal, P., John, A. V., Dorfman, A. L., Grifka, R. Yard., and Figueroa, C. A. (2017). Reproducing patient-specific hemodynamics in the blalock–taussig apportionment using a flexible multi-domain simulation framework: applications for optimal shunt design. Front. Pediatr. 5:78. doi: ten.3389/fped.2017.00078
PubMed Abstract | CrossRef Full Text | Google Scholar
Arthurs, C. J., Khlebnikov, R., Melville, A., Marčan, G., Gomez, A., Dillon-Irish potato, D., et al. (2020). Ruby-red: an open up-source software framework for cardiovascular integrated modelling and simulation. bioRxiv [preprint] doi: x.1101/2020.10.fourteen.339960
CrossRef Full Text | Google Scholar
Baker, C. Eastward., Corsini, C., Cosentino, D., Dubini, G., Pennati, G., Migliavacca, F., et al. (2013). Effects of pulmonary artery banding and retrograde aortic arch obstruction on the hybrid palliation of hypoplastic left middle syndrome. J. Thorac. Cardiovasc. Surg. 146, 1341–1348. doi: 10.1016/j.jtcvs.2013.01.038
PubMed Abstract | CrossRef Full Text | Google Scholar
Ballweg, J., Dominguez, T. Due east., Ravishankar, C., Kreutzer, J., Marino, B. S., Bird, Thou., et al. (2005). A contemporary comparison of the effect of shunt type in hypoplastic left centre syndrome on the hemodynamics and effect at stage 2 reconstruction. J. Thorac. Cardiovasc. Surg. 134, 297–303. doi: 10.1016/j.jtcvs.2007.02.046
PubMed Abstruse | CrossRef Full Text | Google Scholar
Bellsham-Revell, H. R., Tibby, S. Chiliad., Bell, A. J., Witter, T., Simpson, J., Beerbaum, P., et al. (2013). Serial magnetic resonance imaging in hypoplastic left heart syndrome gives valuable insight into ventricular and vascular adaptation. J. Am. Coll. Cardiol. 61, 561–570. doi: 10.1016/j.jacc.2012.xi.016
PubMed Abstract | CrossRef Total Text | Google Scholar
Borenstein, M., Hedges, L. V., Higgins, J. P. T., and Rothstein, H. R. (2010). A bones introduction to fixed-event and random-furnishings models for meta-analysis. Res. Synth. Methods 1, 97–111. doi: 10.1002/jrsm.12
PubMed Abstract | CrossRef Full Text | Google Scholar
Bove, Due east. Fifty., De Leval, G. R., Migliavacca, F., Guadagni, G., and Dubini, G. (2003). Computational fluid dynamics in the evaluation of hemodynamic performance of cavopulmonary connections after the Norwood procedure for hypoplastic left heart syndrome. J. Thorac. Cardiovasc. Surg. 126, 1040–1047. doi: x.1016/s0022-5223(03)00698-6
CrossRef Full Text | Google Scholar
Cardis, B. M., Fyfe, D. A., and Mahle, W. T. (2006). Elastic properties of the reconstructed aorta in hypoplastic left eye syndrome. Ann. Thorac. Surg. 81, 988–991. doi: 10.1016/j.athoracsur.2005.09.065
PubMed Abstract | CrossRef Total Text | Google Scholar
Ceballos, A., Argueta-Morales, I. R., Divo, E., Osorio, R., Caldarone, C. A., Kassab, A. J., et al. (2012). Computational analysis of hybrid Norwood apportionment with distal aortic arch obstruction and reverse blalock-taussig shunt. Ann. Thorac. Surg. 94, 1540–1550. doi: 10.1016/j.athoracsur.2012.06.043
PubMed Abstruse | CrossRef Full Text | Google Scholar
Cua, C. L., Thiagarajan, R. R., Gauvreau, G., Lai, Fifty., Costello, J. Grand., Wessel, D. L., et al. (2006). Early postoperative outcomes in a serial of infants with hypoplastic left heart syndrome undergoing stage I palliation performance with either modified Blalock-Taussig shunt or right ventricle to pulmonary artery conduit. Pediatr. Crit. Intendance Med. 7, 238–244. doi: ten.1097/01.pcc.0000201003.38320.63
CrossRef Full Text | Google Scholar
Dobrin, P. B., Mirande, R., Kang, S., Dong, Q. S., and Mrkvicka, R. (1998). Mechanics of end-to-end artery-to-PTFE graft anastomoses. Ann. Vasc. Surg. 12, 317–323. doi: 10.1007/s100169900161
PubMed Abstract | CrossRef Full Text | Google Scholar
Dolan, J. Grand., Kolega, J., and Meng, H. (2013). Loftier wall shear stress and spatial gradients in vascular pathology: a review. Ann. Biomed. Eng. 41, 1411–1427. doi: 10.1007/s10439-012-0695-0
PubMed Abstract | CrossRef Total Text | Google Scholar
Figueroa, C. A., Vignon-Clementel, I. Eastward., Jansen, K. Due east., Hughes, T. J. R., and Taylor, C. A. (2006). A coupled momentum method for modeling blood menstruation in three-dimensional deformable arteries. Comput. Methods Appl. Mech. Eng. 195, 5685–5706. doi: x.1016/j.jcp.2012.09.016
PubMed Abstruse | CrossRef Full Text | Google Scholar
Fiore, A., Tobin, C., Saadeh, J., Rahimi, M., Kim, East., and Schowengerdt, K. (2011). A comparison of the modified blalock-taussig shunt with the correct ventricle-to-pulmonary artery conduit. Ann. Thorac. Surg. 91, 1479–1484. doi: 10.1016/j.athoracsur.2010.xi.062
PubMed Abstract | CrossRef Full Text | Google Scholar
Fumero, R., Migliavacca, F., Hsia, T.-Y., Dubini, Thousand., de Leval, M. R., Pietrabissa, R., et al. (2017). Modeling of the Norwood apportionment: furnishings of shunt size, vascular resistances, and center charge per unit. Am. J. Physiol. Circ. Physiol. 280, H2076–H2086. doi: 10.1152/ajpheart.2001.280.v.H2076
PubMed Abstract | CrossRef Full Text | Google Scholar
Ghanayem, N. S., Hoffman, G. K., Mussatto, One thousand. A., Frommelt, G. A., Cava, J. R., Mitchell, Thou. East., et al. (2010). Perioperative monitoring in high-risk infants after stage 1 palliation of univentricular built heart disease. J. Thorac. Cardiovasc. Surg. 140, 857–863. doi: 10.1016/j.jtcvs.2010.05.002
PubMed Abstruse | CrossRef Full Text | Google Scholar
Gladman, Grand., McCrindle, B. Due west., Williams, Westward. One thousand., Freedom, R. M., and Benson, L. Northward. (1997). The modified Blalock-Taussig shunt: clinical impact and morbidity in Fallot's tetralogy in the current era. J. Thorac. Cardiovasc. Surg. 114, 25–thirty. doi: 10.1016/S0022-5223(97)70113-2
CrossRef Full Text | Google Scholar
Griselli, G., Mcguirk, S. P., Ofoe, V., Stu, O., Wright, J. 1000. C., Giovanni, J. Five., et al. (2006). Fate of pulmonary arteries post-obit Norwood process. Eur. J. Cardiothorac. Surg. 30, 930–935. doi: 10.1016/j.ejcts.2006.08.007
PubMed Abstract | CrossRef Full Text | Google Scholar
Hirai, T., Sasayama, Southward., Kawasaki, T., and Yagi, S. I. (1989). Stiffness of systemic arteries in patients with myocardial infarction. a noninvasive method to predict severity of coronary atherosclerosis. Apportionment 80, 78–86. doi: x.1161/01.cir.lxxx.1.78
CrossRef Full Text | Google Scholar
Hornik, C. P., He, X., Jacobs, J. P., Li, J. Due south., Peterson, D., and Pasquali, Southward. K. (2012). Complications afterwards the Norwood operation: an analysis of the STS built heart surgery database. Ann. Thorac. Surg. 92, 1734–1740. doi: 10.1016/j.athoracsur.2011.05.100
PubMed Abstract | CrossRef Full Text | Google Scholar
Hozo, Due south. P., Djulbegovic, B., and Hozo, I. (2005). Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 5:13.
Google Scholar
Itatani, K., Miyaji, K., Qian, Y., Liu, J. 50., Miyakoshi, T., Murakami, A., et al. (2012). Influence of surgical arch reconstruction methods on single ventricle workload in the Norwood procedure. J. Thorac. Cardiovasc. Surg. 144, 130–138. doi: 10.1016/j.jtcvs.2011.08.013
PubMed Abstract | CrossRef Full Text | Google Scholar
Jonas, R. A., Hansen, D. D., Cook, Due north., and Wessel, D. (1994). Anatomic subtype and survival after reconstructive operation for hypoplastic left heart syndrome. J. Thorac. Cardiovasc. Surg. 107, 1121–1128. doi: ten.1016/s0022-5223(94)70389-2
CrossRef Full Text | Google Scholar
Kawada, Thou. (2008). Pulmonary artery configuration after the Norwood procedure for hypoplastic left heart syndrome. Gen. Thorac. Cardiovasc. Surg. 56, 61–62.
Google Scholar
Kelleher, D. K., Laussen, P., Teixeira-Pinto, A., and Duggan, C. (2006). Growth and correlates of nutritional status among infants with hypoplastic left heart syndrome (HLHS) afterwards stage 1 Norwood procedure. Nutrition 22, 237–244. doi: 10.1016/j.nut.2005.06.008
PubMed Abstract | CrossRef Full Text | Google Scholar
Lau, One thousand. D., and Figueroa, C. A. (2015). Simulation of brusque-term pressure regulation during the tilt test in a coupled 3D–0D closed-loop model of the circulation. Biomech. Model. Mechanobiol. fourteen, 915–929. doi: 10.1007/s10237-014-0645-x
PubMed Abstract | CrossRef Full Text | Google Scholar
Les, A. S., Shadden, Due south. C., Figueroa, C. A., Park, J. M., Tedesco, K. M., Herfkens, R. J., et al. (2010). Quantification of hemodynamics in abdominal aortic aneurysms during balance and exercise using magnetic resonance imaging and computational fluid dynamics. Ann. Biomed. Eng. 38, 1288–1313. doi: ten.1007/s10439-010-9949-10
PubMed Abstruse | CrossRef Full Text | Google Scholar
Lucas, R., Geme, J., Anderson, R., Adams, P., and Ferguson, D. (1961). Maturation of the pulmonary vascular bed. Am. J. Dis. Kid. 101, 467–475. doi: 10.1001/archpedi.1961.04020050057010
CrossRef Total Text | Google Scholar
Machii, Yard., and Becker, A. E. (1997). Morphologic features of the normal aortic curvation in neonates, infants, and children pertinent to growth. Ann. Thorac. Surg. 64, 511–555. doi: 10.1016/S0003-4975(97)00445-ane
CrossRef Full Text | Google Scholar
Maher, K. O., Pizarro, C., Gidding, Southward. S., Januszewska, K., Malec, E., Norwood, W. I., et al. (2003). Hemodynamic profile after the Norwood procedure with correct ventricle to pulmonary artery conduit. Circulation 108, 782–784. doi: 10.1161/01.cir.0000087338.09589.21
CrossRef Total Text | Google Scholar
Mahle, West. T., Cuadrado, A. R., and Tam, 5. K. H. (2003). Early experience with a modified Norwood procedure using correct ventricle to pulmonary artery conduit. Ann. Thorac. Surg. 76, 1084–1088. doi: 10.1016/s0003-4975(03)00343-half-dozen
CrossRef Full Text | Google Scholar
Mahle, West. T., Rychik, J., Weinberg, P. M., and Cohen, Grand. S. (1998). Growth characteristics of the aortic arch after the Norwood operation. J. Am. Coll. Cardiol. 32, 1951–1954. doi: 10.1016/s0735-1097(98)00457-4
CrossRef Total Text | Google Scholar
Mahle, W. T., Spray, T. L., Wernovsky, Grand., Gaynor, J. West., and Iii, B. J. C. (2000). Survival after reconstructive surgery for hypoplastic left heart syndrome. Circulation 102, 136–141.
Google Scholar
Mai, C. T., Rickard, R., Anderson, P., Mason, C. A., Parker, S. E., Meyer, R. E., et al. (2010). Updated national nascence prevalence estimates for selected birth defects in the United States, 2004-2006. Birth Defects Res. A Clin. Mol. Teratol. 88, 1008–1016. doi: x.1002/bdra.20735
PubMed Abstract | CrossRef Full Text | Google Scholar
Mair, R., Tulzer, G., Sames, E., Gitter, R., Lechner, East., Steiner, J., et al. (2003). Correct ventricular to pulmonary artery conduit instead of modified Blalock-Taussig shunt improves postoperative hemodynamics in newborns subsequently the Norwood performance. J. Thorac. Cardiovasc. Surg. 126, 1378–1384. doi: ten.1016/s0022-5223(03)00389-1
CrossRef Full Text | Google Scholar
Malec, E., Januszewska, Thou., Kolcz, J., and Mroczek, T. (2003). Right ventricle-to-pulmonary artery shunt versus modified Blalock-Taussig shunt in the Norwood process for hypoplastic left heart syndrome - Influence on early and late haemodynamic status. Eur. J. CardioThorac. Surg. 23, 728–734. doi: ten.1016/s1010-7940(03)00072-1
CrossRef Full Text | Google Scholar
Migliavacca, F., Pennati, Thou., Di Martino, E., Dubini, G., and Pietrabissa, R. (2002). Pressure drops in a distensible model of terminate-to-side anastomosis in systemic-to-pulmonary shunts. Comput. Methods Biomech. Biomed. Eng. 5, 243–248. doi: 10.1080/10255840290010689
PubMed Abstract | CrossRef Full Text | Google Scholar
Moghadam, Yard., Migliavacca, F., Vignon-Clementel, I., Hsia, T.-Y., and Marsden, A. (2012). Optimization of shunt placement for the Norwood surgery using multi-domain modeling. J. Biomech. Eng. 134:051002. doi: ten.1115/one.4006814
CrossRef Full Text | Google Scholar
Pettersen, M. D., Du, W., Skeens, M. E., and Humes, R. A. (2008). Regression equations for calculation of Z scores of cardiac structures in a big cohort of healthy infants, children, and adolescents: an echocardiographic written report. J. Am. Soc. Echocardiogr. 21, 922–934. doi: 10.1016/j.echo.2008.02.006
PubMed Abstract | CrossRef Total Text | Google Scholar
Pizarro, C., and Norwood, West. I. (2003). Correct ventricle to pulmonary artery conduit has a favorable touch on on postoperative physiology subsequently stage I Norwood: preliminary results q. Eur. J. Cardiothorac. Surg. 23, 991–995. doi: 10.1016/s1010-7940(03)00158-1
CrossRef Full Text | Google Scholar
Pizarro, C., Malec, Eastward., Maher, K. O., Januszewska, K., Gidding, S. Southward., Murdison, K. A., et al. (2003). Right ventricle to pulmonary artery conduit improves outcome after stage I Norwood for hypoplastic left centre syndrome. Circulation 108, 155–160. doi: x.1161/01.cir.0000087390.94142.1d
CrossRef Full Text | Google Scholar
Qian, Y., Liu, J. L., Itatani, Thousand., Miyaji, Chiliad., and Umezu, One thousand. (2010). Computational hemodynamic assay in built heart illness: simulation of the Norwood procedure. Ann. Biomed. Eng. 38, 2302–2313. doi: 10.1007/s10439-010-9978-v
PubMed Abstract | CrossRef Full Text | Google Scholar
Roccabianca, Southward., Figueroa, C. A., Tellides, G., and Humphrey, J. D. (2014). Quantification of regional differences in aortic stiffness in the aging human. J. Mech. Behav. Biomed. Mater. 29, 618–634. doi: 10.1016/j.jmbbm.2013.01.026
PubMed Abstruse | CrossRef Full Text | Google Scholar
Rychik, J., Bush, D. M., Spray, T. L., Gaynor, J. W., and Wernovsky, G. (2000). Cess of pulmonary/systemic blood flow ratio after first-stage palliation for hypoplastic left eye syndrome: evolution of a new alphabetize with the apply of doppler echocardiography. J. Thorac. Cardiovasc. Surg. 120, 81–87.
Google Scholar
Rychik, J., Gaynor, J. West., Wernovsky, M., Bush, D. M., and Spray, T. L. (2002). Assessment of pulmonary/systemic claret menstruum ratio after commencement-stage palliation for hypoplastic left eye syndrome. J. Thorac. Cardiovasc. Surg. 120, 81–87. doi: 10.1067/mtc.2000.106840
PubMed Abstract | CrossRef Full Text | Google Scholar
Sahni, O., Müller, J., Jansen, 1000. E., Shephard, M. S., and Taylor, C. A. (2006). Efficient anisotropic adaptive discretization of the cardiovascular arrangement. Comput. Methods Appl. Mech. Eng. 195, 5634–5655. doi: ten.1016/j.cma.2005.10.018
CrossRef Full Text | Google Scholar
Sano, Southward., Ishino, Yard., Kawada, M., and Honjo, O. (2004). Right ventricle-pulmonary artery shunt in first-stage palliation of hypoplastic left eye syndrome. Pediatr. Menu. Surg. Annu. 7, 22–31. doi: x.1053/j.pcsu.2004.02.023
PubMed Abstract | CrossRef Total Text | Google Scholar
Senzaki, H., Akagi, M., Hishi, T., Ishizawa, A., Yanagisawa, Thousand., Masutani, S., et al. (2002). Age-associated changes in arterial elastic properties in children. Eur. J. Pediatr. 161, 547–551. doi: ten.1007/s00431-002-1025-6
PubMed Abstract | CrossRef Total Text | Google Scholar
Silva Vieira, M., Arthurs, C. J., Hussain, T., Razavi, R., and Figueroa, C. A. (2018). Patient-specific modeling of correct coronary circulation vulnerability post-liver transplant in Alagille's syndrome. PLoS One 13:e0205829. doi: 10.1371/journal.pone.0205829
PubMed Abstract | CrossRef Full Text | Google Scholar
Tossas-Betancourt, C., van Bakel, T. Thousand. J., Arthurs, C. J., Coleman, D. M., Eliason, J. L., Figueroa, C. A., et al. (2020). Computational analysis of renal artery period characteristics by modeling aortoplasty and aortic bypass interventions for abdominal aortic coarctation. J. Vasc. Surg. 71, 505–516.e4. doi: 10.1016/j.jvs.2019.02.063
PubMed Abstract | CrossRef Full Text | Google Scholar
Tweddell, J. Due south., Hoffman, G. M., Mussatto, G. A., Fedderly, R. T., Berger, Due south., and Jaquiss, R. B. D. (2002). Improved survival of patients undergoing palliation of hypoplastic left heart syndrome: lessons learned from 115 sequent patients. Apportionment 106, I82–I89.
Google Scholar
van Meurs-van Woezik, H., Klein, H. W., Markus-Silvis, 50., and Krediet, P. (1983). Comparing of the growth of the tunica media of the ascending aorta, aortic isthmus and descending aorta in infants and children. J. Anat. 136, 273–281.
Google Scholar
Vignon-Clementel, I. Due east., Figueroa, C. A., Jansen, G. Due east., and Taylor, C. A. (2010). Outflow purlieus weather for 3D simulations of non-periodic blood menstruation and pressure fields in deformable arteries. Comput. Methods Biomech. Biomed. Eng. 13, 625–640. doi: 10.1080/10255840903413565
PubMed Abstract | CrossRef Total Text | Google Scholar
Wells, W. J., Yu, R. J., Batra, A. S., Monforte, H., Sintek, C., and Starnes, V. A. (2005). Obstacle in modified Blalock shunts: a quantitative analysis with clinical correlation. Ann. Thorac. Surg. 79, 2072–2076. doi: 10.1016/j.athoracsur.2004.12.050
PubMed Abstract | CrossRef Full Text | Google Scholar
Xiao, N., Alastruey, J., and Figueroa, C. A. (2013). Multi-physics in biomechanical modeling a systematic comparison betwixt i-D and 3-D hemodynamics in compliant arterial models. Int. J. Number Method Biomed. Eng. 30, 204–231. doi: ten.1002/cnm.2598
PubMed Abstract | CrossRef Full Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fphys.2021.603040/full
0 Response to "When Does 4cs in Norwood"
Post a Comment